CMA 30 Linear Microdialysis Probes
The CMA 30 Linear Microdialysis Probe is ideal for peripheral tissues such as skin, muscle, heart, adipose tissue, liver, eye, pancreas and other organs as well as tumors.
- Tailored for dialysis in peripheral tissues as well as for spinal cord and tumors
- Soft and flexible construction
- Can be sterilized with ethylene oxide
- Available membrane: Cuprophane, 6 kDa MWCO
- Membrane lengths - 10 mm
The CMA 30 Linear Microdialysis Probe is ideal for peripheral tissues such as skin, muscle, heart, adipose tissue, liver, eye, pancreas and other organs as well as tumors.
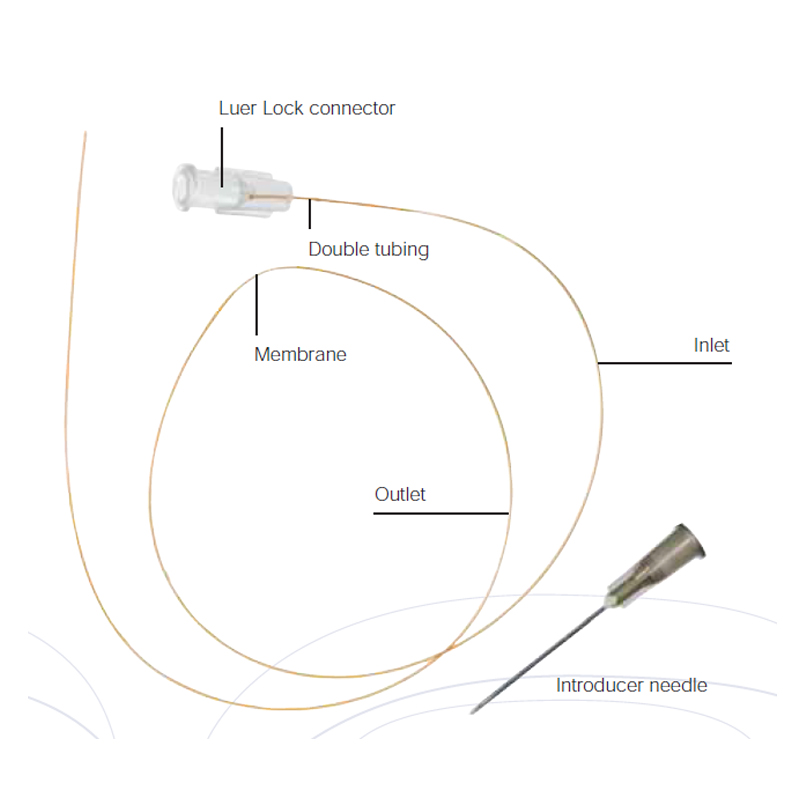
The probe consists of a tubing in which the middle part has a 10 mm window with a Cuprophane membrane, 6,000 Daltons cut off. Along the membrane, in this window, a thin part of the tubing remains to increase the stability and also to secure the membrane during withdrawal from the tissue. The probe is easy to implant with an introducer needle that is included in the package.
The inlet of the probe has a Luer lock connector, which can be attached to a single use syringe. Or, if a glass syringe and tubing adapter will be used, this connector can easily be cut off and discarded.
One package contains 4 probes, each in an individual pouch for easy handling.
The probes can be sterilized in its package with ethylene oxide.
| Membrane Length | 10 mm | |
| Membrane OD | 0.24 mm | |
| Membrane Material | Cuprophane | |
| Molecular Weight Cut-Off | 6 kD | |
| Tubing Inner Diameter | 0.28 mm | |
| Tubing Outer Diameter | 0.38 mm | |
| Inlet and Outlet Lengths | 245 mm | |
| Double Tubing OD | 0.63 mm | |
| Introducer Length | 26 mm | |
| Introducer Diameter | 0.7, 22 G |
Selected Recent Publications
Prathipati, P., Jackson, D. & Jackson, K., 2017. Evaluating the significance of hypoglycemia in promoting insulin induced hypertension by using a glucose clamp model. International Journal of Medicine, 5(1), pp.22–27.
Joosen, M.J.A. et al., 2017. The impact of skin decontamination on the time window for effective treatment of percutaneous VX exposure. Chemico-Biological Interactions, 267, pp.48–56.
Yang, Y. et al., 2016. In situ eNOS/NO up-regulation—a simple and effective therapeutic strategy for diabetic skin ulcer. Scientific Reports, 6. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4958962/.
Alsulimani, H.H., Kim, J. & Sani, S.N., 2016. Microdialysis-directed Intra-tumor Pharmacokinetic Modeling of Methotrexate in Mice and Humans. Journal of Pharmacy & Pharmaceutical Sciences, 19(2), pp.239–251.
Xie, F. et al., 2016. Transdermal permeation of drugs with differing lipophilicity: Effect of penetration enhancer camphor. International Journal of Pharmaceutics, 507(1–2), pp.90–101.
Wang, G. et al., 2015. Simultaneous Determination of Four Substances in Plasma and Dermal Microdialysates of Guinea Pig after Different Acupoints Administration of Fufang Baijiezi Gel. Chinese Herbal Medicines, 7(4), pp.365–370.
Wan, T. et al., 2015. Microemulsion based gel for topical dermal delivery of pseudolaric acid B: In vitro and in vivo evaluation. International Journal of Pharmaceutics, 493(1–2), pp.111–120.
Wei, Y. et al., 2015. Effect of glycyrrhizic acid on rhein renal penetration: a microdialysis study in rats. Xenobiotica, 45(12), pp.1116–1121.
Naylor, L.H. et al., 2014. Acute hyperglycaemia does not alter nitric oxide-mediated microvascular function in the skin of adolescents with type 1 diabetes. European Journal of Applied Physiology, 114(2), pp.435– 441.
Li, S.-S. et al., 2014. The percutaneous permeability and absorption of dexamethasone esters in diabetic rats: a preliminary study. Drug Delivery, 21(1), pp.17–25.
Visit our Publications page for a complete listing of CMA 30 publications.


